
Genetics Basics
Genes (http://www.dnalife.healthcare/genetics-in-practice/)
Genes are made up of DNA (deoxyribonucleic acid) and are the template to make proteins. We have two copies of each gene, one inherited from each parent. Human DNA consists of over 3 billion base pairs, 99% of which are identical between individuals. The remaining 1% contains small variations known as SNPs (single nucleotide polymorphisms).
A SNP (single nucleotide polymorphism) is a genetic variation that results in a single base change in the DNA sequence between two individuals.
- SNPs can have a profound effect on the functioning of the genes in which they are found. This in turn affects the biological pathway in which the gene is active, affecting metabolic functions that are important for maintaining a state of health.
- Knowledge of these SNPs offers a powerful health advantage, enabling the trained healthcare practitioner to prescribe precise lifestyle and nutritional recommendations aimed at compensating for the genetic variants.
- A criterion for inclusion of a specific SNP in one of our tests is that there must be an intervention which has been proven to modify the effect of the SNPs that we identify.

Dr. Sadak is trained and certified in administering and interpreting the following genetic tests:
DNA Mind (Sample report)
A sound mind in a sound body
According to research, neuropsychiatric disorders account for up to 25% of all disability-adjusted life years. Whilst the heritability of these mental disorders is significant, environmental factors also factor into their development. Genetic variations involved in key biological processes that contribute toward the risk of development of mental health disorders may give insights to the prevention, diagnosis and treatment of the disease.
By understanding an individual’s unique genetic makeup and predispositions, healthcare clinical professionals can better in developing strategies for individuals suffering from, or at risk of, mental health disorders.
The DNA Mind Report Provides:
- Identification of the level of impact of any genetic variants
- An explanation of their impact on the specific biochemical area
- An explanation of each area’s impact on mental health
Taking the test may help you, and/or your patients, understand ways that may avoid the onset of a range of neurological disorders.
The DNA Mind test analyses 30 genes which have been shown to have significant associations with key mental health disorders, and reports on the following areas:
- Neurodegenerative disorders
- Alzheimer’s disease, dementia, cognitive decline
- Addictive behaviour
- A risk for alcohol, nicotine, cannabis & opioid dependence; Psychosis response from cannabis use; eating disorders (binge eating)
- Adrenaline-seeking
- Risk-taking behaviour
- Mood disorders
- Depression, bipolar, anxiety and post-traumatic stress
DNA Health (sample report)
The DNA Health test is designed to optimise wellbeing and health by personalising lifestyle and diet choices and, where necessary, using supplements tailored to offset any particular nutritional deficit based on specific gene variants. The DNA Health approach assists the healthcare practitioner in establishing the optimal nutrition necessary for good health, longevity and disease risk mitigation.DNA Health tests for 36 gene variants involved in the following biological processes that have been linked to risk for diseases of lifestyle:
Lipid metabolism
Nutrition and lifestyle factors such as exercise, dietary fats and carbohydrates impact lipid metabolism and lipid levels, which may contribute risk of developing cardiovascular disease (CVD). However, these influences are potentially modulated by gene variations that play a role in lipid metabolism.
Single-gene defects affect a relatively small subset (5-10%) of patients at high risk of premature coronary heart disease, while multiple gene variations with minor effects contribute to CVD risk in the vast majority of individuals in the general population. Such polygenic effects depend predominantly on environmental influences.
Most premature cardiovascular deaths can be prevented if action is taken to avoid or modify external exposures that may cause a genetic predisposition to become clinically relevant.
The lipid metabolism panel analyses SNPs on the following genes:
- CETP
- LPL
- APOC3
- APOE
Bone Health & risk for osteoporosis
- Osteoporosis is a complex disease characterized by decreased bone mass, deterioration of bone tissue and increased risk of fractures. Family and twin studies have established a strong genetic contribution to the aetiology of osteoporosis, and 60-70% of the variability in bone mineral mass or bone mineral density (BMD) can be accounted for by genetic variation and variations in diet. The bone health panel analyses SNPs on the following genes:
- VDR
- COL1A1
Methylation and risk for cancer
-
B vitamins provide building blocks for growing cells, which are constantly being renewed, and play an important role in many physiological processes. B vitamins also supply some of the chemicals necessary for protecting our genes, so that our DNA doesn’t accumulate damage from the wear and tear in the daily lives of our cells. These vitamins – including folate, vitamins B6 and B12 – help make new DNA for cells that are constantly growing and renewing themselves. Folate is also involved in turning many genes on and off, and also helps repair DNA. The process of DNA repair is called methylation. Although B vitamins are only required in small amounts, they are crucial for methylation and in producing new DNA. SNPs found on genes that regulate B-vitamin associated biochemical pathways may influence and individual’s requirements for these essential nutrients.
The Methylation Panel analyses SNPs on the following genes:
- MTHFR
- MTR
- MTRR
- CBS
- COMT
Inflammation and oxidative stress
The biotransformation or detoxification is governed by two main phases. Phase 1 detoxification is known as the ‘activation’ phase, where enzymes activate the substance that needs to be removed, allowing the next phase to proceed. Phase I enzymes must exhibit just the right amount of activity for the detoxification process to be effective. Activated compounds in phase I are potentially harmful.
The enzymes that take over from phase I are called ‘excretors’ because they catalyze reactions leading to the excretion of toxins from the body. These enzymes bind the chemical compound glutathione to the ‘active’ toxins from phase I, making them water soluble so they can be excreted through sweat or urine. An imbalance between phase 1 and phase 2 detoxification due to the presence of SNPs is associated with increased risk for DNA damage, cancer and other diseases.
The Detoxification Panel analyses SNPs on the following genes:
- CYP1A1
- GSTM1
- GSTT1
- GSTP1
- NQO1
Free radicals are a normal by-product of the body’s energy-generating biochemical processes. They are highly reactive with other molecules, and can damage DNA, proteins and cellular membranes. Anti-oxidants are free radical scavengers that interact with the free radical to ensure it is no longer a reactive molecule.
Anti-oxidants are found naturally in the body in the form of enzymes, but can also be consumed in a wide variety of foods, especially vegetables and fruits. Increased risk of oxidative damage can occur in the presence of certain SNPs and in combination with an unhealthy lifestyle.
The Oxidative Stress Panel analyses SNPs on the following genes:
- SOD2
- eNOS
Detoxification
The biotransformation or detoxification is governed by two main phases. Phase 1 detoxification is known as the ‘activation’ phase, where enzymes activate the substance that needs to be removed, allowing the next phase to proceed. Phase I enzymes must exhibit just the right amount of activity for the detoxification process to be effective. Activated compounds in phase I are potentially harmful.
The enzymes that take over from phase I are called ‘excretors’ because they catalyze reactions leading to the excretion of toxins from the body. These enzymes bind the chemical compound glutathione to the ‘active’ toxins from phase I, making them water soluble so they can be excreted through sweat or urine. An imbalance between phase 1 and phase 2 detoxification due to the presence of SNPs is associated with increased risk for DNA damage, cancer and other diseases.
The Detoxification Panel analyses SNPs on the following genes:
- CYP1A1
- GSTM1
- GSTT1
- GSTP1
- NQO1
Insulin Sensitivity and risk for diabetes
Insulin is a hormone that stimulates the uptake of glucose from the diet into the blood. Those with lowered sensitivity to insulin have a limited ability to respond to the hormone’s action. The scientific literature suggests that insulin insensitivity or resistance may play an important role in some of the most common disorders including, obesity and type 2 diabetes. Clustering of Type 2 Diabetes in certain families’ points to a strong genetic background for the disease, however environmental factors such as obesity and a sedentary lifestyle are usually required to unmask the genes. The I nsulin Sensitivity Panel analyses SNPs on the following genes:
- SLC2A2
- TCF7L2
- FTO
- PPARG
Food Responsiveness
- Lactose intolerance
- Caffeine processing
- Salt sensitivity
- Blood pressure
- Iron overload disorders
Particular nutrients and certain food components in different foodstuffs can affect individuals in different ways. With new research coming to light in this area, specific genes can be tested to give more insight to how an individual might respond to a particular food component. The areas of food responsiveness covered in this panel include: Lactose intolerance, Polyunsaturated Fat (PUFA) metabolism, caffeine metabolism and salt sensitivity and blood pressure, and iron overload.
The Food Responsiveness Panel analyses SNPs on the following genes:
- FADS1
- MCM6
- CYP1A2
- ACE
- AGT

DNA Diet (sample report)
DNA Diet is designed to assist the healthcare practitioner in the design of a personalised weight management plan based on individual genetic differences.DNA Diet also provides additional insight into how each individual reacts to carbohydrates, saturated fats and intensity of exercise, allowing further personalization of your eating plan to suit your needs. DNA Diet tests a number of well-researched gene variations that impact metabolism, absorption and storage of fats and carbohydrates, as well as eating behaviour. We have analysed these SNPs to understand how an individual’s genetic profile will impact their response to what we believe to be the three most effective healthy eating plans. i.e. Low fat, Mediterranean, and Low Carbohydrate. Research suggests that individuals do respond differently to different food combinations and no ONE way of eating is right for everyone.
DNA Oestrogen (sample report)
80% of breast cancer occurs in women with no family history. Oestrogen gene testing may help you lessen your risk. Research has shown that an increased lifetime exposure to oestrogen is a strong risk factor in the development of breast cancer. DNA Oestrogen tests for gene variants that have been shown to have an impact on how oestrogen is processed in your body and if the processing of oestrogen and related compounds is efficient and healthy. Oestrogen affects the function of a number of target tissues. Guiding the personalisation of diet, hormone and nutritional supplement recommendations, based on the insight gained from the DNA Oestrogen test, is of benefit to men and women who suffer from numerous oestrogen-dominant conditions. DNA Oestrogen reports on the following areas:
- Variations in key genes involved in metabolizing oestrogen and related compounds
- Intervention strategies for carriers of high-risk genetic variations
- Personal risk factors associated with HRT, Oral contraceptives, bio-identical supplementation and in vitro fertilization
DNA Sport (sample report)
DNA Sport examines various biological areas that impact training responsiveness and sporting performance. These include soft tissue remodeling, inflammation, blood flow and respiration, energy production and fuel metabolism.
Athletic success results firstly from personalising a training strategy that optimises your genetic potential, and secondly choosing the right lifestyle, nutrition and environmental interactions to optimally express your inherited genes.
DNA Sport reports on the following areas:
- structural integrity of soft tissues
- inflammation & oxidative stress
- blood flow & respiration
- energy during exercise
- fuel during exercise
- caffeine metabolism
- muscle and bone composition
- aerobic capacity
- power/strength potential
Knowledge of variations in genes involved in these biological areas can be used to personalise a training program in order to gain as much as possible from sessions by exploiting potential advantages, as well as to identify weaknesses that need to be worked on. This insight can also be used to make appropriate training and nutrition choices in an attempt to prevent injury as well as optimise recovery in order to perform at one’s best.
PHARMACOGENOMICS
Medcheck (sample report)
Pharmacogenomics empowers the doctor to provide specific medical interventions, based on the patient’s genetic profile and replaces the current trial and error approach to drug therapy.
As a worldwide leader in the field of pharmacogenomics, DNAlysis is able to test an individual’s genetics to determine how a patient will respond to medication. How a patient responds to medications is determined by the genes that govern drug metabolism, and pharmacogenomics empowers the doctor to provide specific medical interventions, based on the patient’s genetic profile and replaces the current trial and error approach to drug therapy.
At DNAlife, we test how patients’ genes metabolise, transport, and bind specific prescription drugs; this informs physicians to prescribe personalised regimes of the most effective compounds.
Patients most likely to benefit from Medcheck testing include those:
- Experiencing side effects of specific medications
- Not responding to specific medications
- Requiring doses outside the recommended range
- Planning to start on a new medication
DRUG CLASSES COVERED
- Cardiovascular Medications
- Angiotensin II Receptor Antagonists
- Antianginal Agents
- Antiarrhythmics
- Anticoagulants
- Antiplatelets
- Beta Blockers
- Diuretics
- Statins
- Psychotropic Medications
- Antiaddictives
- Anti-ADHD Agents
- Anticonvulsants
- Antidementia Agents
- Antidepressants
- Antipsychotics
- Benzodiazepines
- Mood Stabilizers
- Pain Medications
- Fibromyalgia Agents
- Muscle Relaxants
- NSAIDs
- Opioids
Genetic Testing in Dementia
Excellent Information Resource/Forum
ApoE4.info, Inc. Mission - dedicated to understanding the APOE-ε4 gene and how it affects health. By:
- providing basic information about the APOE-ε4 gene
- running an active forum and Facebook page
- acting as a contact point for anyone interested in the APOE-ε4 gene
- pursuing research about the APOE-ε4 allele to mitigate related negative health effects including but not limited to Alzheimer’s disease and cardiovascular disease
- connecting researchers and carriers of the gene
- organizing regular APOE-ε4 Conferences
- working with the media to inform and educate health professionals and the public about the APOE-ε4 gene
Inform Yourself
Learn the basics. We recommend our primer as the ideal starting place for your journey. Authored by a member physician who carries two copies of the APOE-ε4 allele, the primer thread offers accessible science background and prioritized, sensible preventative measures.
Consider testing. Learning one’s APOE-ε4 status can be life changing for you and your loved ones. Our Thinking About Testing ? page identifies the issues to consider BEFORE you test.
Read some of our discussions. Poke around the forums t o see what’s being discussed. The level of conversation can range from straightforward sharing of personal experiences and concerns to nuanced discussions about published research. Use our search to look for specific topics.
Join Us
Join the discussion. If you want to take part in the conversation, create a free account f or yourself to become a member. When you join, please introduce yourself. You can share as much or as little as you like. And for tips and tricks to get the most out of our discussion forums, visit this handy guide .
Follow us on Facebook. For occasional summaries of important new research, follow our Facebook page.
WHAT APOE MEANS FOR YOUR HEALTH

Genes are one of many risk factors for dementia. While a quarter of Alzheimer's patients have a strong family history of the disease, only 1% directly inherit a gene mutation that causes early-onset Alzheimer's, also known as familial Alzheimer's disease (FAD) [1] . But another gene called APOE can influence your risk for the more common late-onset type of Alzheimer's.
There are three types of the APOE gene, called alleles: APOE2, E3 and E4. Everyone has two copies of the gene and the combination determines your APOE "genotype"—E2/E2, E2/E3, E2/E4, E3/E3, E3/E4, or E4/E4. The E2 allele is the rarest form of APOE and carrying even one copy appears to reduce the risk of developing Alzheimer's by up to 40%. APOE3 is the most common allele and doesn't seem to influence risk. The APOE4 allele, present in approximately 10-15% of people, increases the risk for Alzheimer's and lowers the age of onset. Having one copy of E4 (E3/E4) can increase your risk by 2 to 3 times while two copies (E4/E4) can increase the risk by 12 times [2] .
Despite this association, the National Institutes of Health only recommends genetic testing for APOE status to advance drug research in clinical trials. APOE4 is just one of many risk factors for dementia and its influence can vary across age, gender, race, and nationality [3] [4] . For example, having one copy of the E4 allele may pose more risk to women while having two copies seems to affect men and women similarly [5] .
To learn more about the genetics of Alzheimer's disease and the contribution of the APOE genes, check out the National Institute on Aging's Alzheimer's Disease Genetics Fact Sheet .
THE BIOLOGY OF APOE
The APOE protein plays many important roles, including the transport of cholesterol across different tissues and cells. The proteins made by varying APOE alleles handle this transport function differently.
Outside the brain, APOE4 can increase the risk of atherosclerosis (i.e., hardening of the arteries) and stroke [4] , which may explain why APOE4 is a risk factor for vascular causes of cognitive impairment and dementia [6] [7] . Inside the brain, APOE helps to clear beta-amyloid, a component of plaques. APOE2 appears to perform this function more effectively than APOE4, with APOE3 in the middle. This difference in beta-amyloid transport represents what scientists call "loss-of-function" toxicity. However, researchers suspect that APOE4 proteins may also have toxic "gain-of-function" activities, such as increased response to stress or injury [4] .
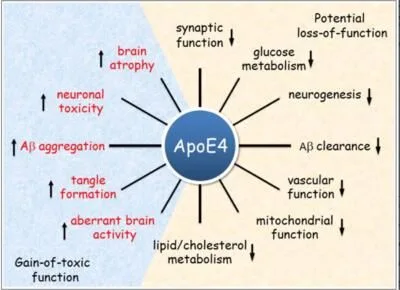
APOE4 may increase the risk of dementia through toxic gain of function and through the loss of normal healthy function. Figure adapted from [4]
APOE4 AND ALZHEIMER'S DRUG DISCOVERY
Some drugs in development (called "structure correctors") may change the physical structure of the APOE4 protein so that it behaves more like the APOE2 protein [8] . Another approach is gene therapy, which attempts to insert APOE2 genes into the brains of people with APOE4 genes [9] . To learn more about these programs and other APOE-related drug discovery programs supported by the Alzheimer's Drug Discovery Foundation, review our research portfolio with a filter for "APOE4."
DOES APOE AFFECT HOW THERAPIES WORK?
Researchers are exploring whether the APOE genotype influences the effects of drugs and other therapies in development for Alzheimer's disease and general cognitive health. Highlights of the scientific research in which a differential effect is possible follows, with links to reports.
Estrogen: Several studies suggest that the side effects of estrogen-containing hormone replacement therapy may be worse in people who carry the APOE4 allele, at least in terms of brain aging and dementia risk. However, the evidence is inconsistent.
Hypertension Management: Effective management of mid-life hypertension is likely to reduce the risk of dementia and cognitive decline in most people. Observational studies suggest that APOE4 carriers might be particularly likely to reap the benefits of effective hypertension management. However, the complex relationships between cardiovascular health, APOE status, and cognition are not well understood.
DHA: Although DHA may be part of a healthy diet for APOE4 carriers, evidence from observational studies, clinical trials, and some preclinical research suggests that it is less likely to protect against dementia or cognitive decline in APOE4 carriers. Some researchers are testing the idea that APOE4 carriers simply need higher doses of DHA because it does not reach their brains as effectively [10] .
Statins: Evidence is mixed on whether statins have different effects on brain health in people who carry at least one APOE4 allele. Several observational studies found that APOE4 allele status had no effect while another suggested different effects on cognition in patients with at least one APOE4 allele.
Nicotine: Although there is no evidence suggesting different Alzheimer's disease benefits from nicotine between APOE4 carriers and non-carriers, some evidence suggests nicotine may be a stronger acute cognitive enhancer in APOE4 carriers than non-carriers.
Cerebrolysin: One clinical trial comparing the Exelon™ patch with cerebrolysin found no difference in response rates in patients with at least one APOE4 allele but a 3-fold higher response rate in patients without an APOE4 allele.
- Bird, T.D., Alzheimer Disease Overview, in GeneReviews(R) , R.A. Pagon, et al., Editors. 1993: Seattle (WA).
- Michaelson, D.M., APOE epsilon4: the most prevalent yet understudied risk factor for Alzheimer's disease . Alzheimers Dement, 2014. 10(6): p. 861-8.
- Farrer, L.A., et al., Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium . JAMA, 1997. 278(16): p. 1349-56.
- Liu, C.C., et al., Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy . Nat Rev Neurol, 2013. 9(2): p. 106-18.
- Riedel, B.C., P.M. Thompson, and R.D. Brinton, Age, APOE and sex: Triad of risk of Alzheimer's disease . J Steroid Biochem Mol Biol, 2016. 160: p. 134-47.
- Dwyer, R., et al., Using Alzgene-like approaches to investigate susceptibility genes for vascular cognitive impairment . J Alzheimers Dis, 2013. 34(1): p. 145-54.
- Sun, J.H., et al., Genetics of Vascular Dementia: Systematic Review and Meta-Analysis . J Alzheimers Dis, 2015. 46(3): p. 611-29.
- Mahley, R.W. and Y. Huang, Small-molecule structure correctors target abnormal protein structure and function: structure corrector rescue of apolipoprotein E4-associated neuropathology . J Med Chem, 2012. 55(21): p. 8997-9008.
- Zhao, L., et al., Intracerebral adeno-associated virus gene delivery of apolipoprotein E2 markedly reduces brain amyloid pathology in Alzheimer's disease mouse models . Neurobiol Aging, 2016. 44: p. 159-72.
- Yassine, H.N., et al., The effect of APOE genotype on the delivery of DHA to cerebrospinal fluid in Alzheimer's disease . Alzheimers Res Ther, 2016. 8: p. 25.
Dr. Penny Dacks was previously the Director of Aging and Alzheimer’s Disease Prevention at the Alzheimer's Drug Discovery Foundation. She was trained in neuroscience at the Mount Sinai School of Medicine, the University of Arizona, and Queen's University (Canada) with individual fellowships from the National Institute of Health, the Evelyn F. McKnight Brain Research Foundation, the ARCS Foundation and the Hilda and Preston Davis Foundation. She has authored over 18 peer-reviewed scientific articles and is a member of the Society for Neuroscience, the Gerontological Society of America, the Endocrine Society and the Association for Women in Science.
For more information go to: https://www.alzdiscovery.org/c...
Other Genetic Mutations in Dementia
https://www.alzforum.org/mutations, Click here to review interactive diagrams
Three genes are associated with autosomal-dominant AD (APP, PSEN1, PSEN2) plus two genes associated with AD by way of genetics or the neuropathology of the encoded protein ( TREM2 and MAPT ). The goal is to provide a comprehensive list of rare variants in these genes, ranging from causative to benign, and to briefly describe any associated clinical and neuropathological features and reported functional effects, as reported in the literature.
APP
The APP gene encodes amyloid precursor protein, a transmembrane protein whose proteolysis gives rise to Aβ peptides. Mutations in APP are associated with Alzheimer's disease and Cerebral Amyloid Angiopathy.
MAPT
MAPT encodes the microtubule associated protein tau, a protein central to Alzheimer’s disease neuropathology. MAPT mutations are not linked to familial forms of AD, but can cause frontotemporal dementia (FTD) and several other tauopathies. The pathogenic mutations, which can be either exonic or intronic, generally alter the relative production of tau isoforms and lead to changes in microtubule assembly and/or the propensity of tau to aggregate.
PSEN1
PSEN1 encodes presenilin-1, a subunit of γ-secretase, the aspartyl protease responsible for Aβ generation. Over 180 mutations in PSEN1 have been reported and mutations in PSEN1 are the most common cause of early onset Alzheimer's disease.
PSEN2
The gene PSEN2 encodes presenilin-2, a subunit of γ-secretase, the aspartyl protease responsible for Aβ generation. Missense mutations in PSEN2 are a rare cause of early onset Alzheimer's disease.
TREM2
TREM2 encodes Triggering Receptor Expressed on Myeloid Cells 2, a transmembrane receptor that modulates microglial activity and survival. TREM2 variants cause Nasu-Hakola disease (NHD), a rare autosomal recessive early-onset dementia, and may modify the risk of developing Alzheimer’s disease (AD), frontotemporal dementia (FTD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS).

